One of my goals for our blog is to show (to the few people who may read this who don't know us personally) that scientists are just like regular people whose job happens to be mixing together clear liquids all day. For this reason, I am pretty interested in DIYbio, a group that aims to make science, and biology in particular, more accessible to people who are interested in viewing the world around them scientifically but maybe don't have a degree in science. [What follows is probably the most serious/boring post we have on here, but believe it or not, we are serious sometimes and this is something I feel is important enough to go into excruciating detail about. Feel free to skip this and go back to looking up cusses in scholarly journals.]
I think it is totally awesome that people who don't have a lot of experience with science think that biology is cool enough to meet at bars to talk about, and it is great that people want to do experiments. However, in the process of demystifying biology so that amateurs can interact with things like bacteria, DNA, and even genomes, DIYbio often ends up pushing "traditional" science further into the abstract ivory tower. This is especially true I think in the discussions around how to do DNA electrophoresis (separating different pieces of DNA based on their size). In seeing what DIYbio is proposing as methods for doing this common lab technique, it is clear that the people who do it every day (grad students, post docs, and technicians working in labs everywhere) haven't done a really good job of explaining how the data that is finally presented to the public is "made"; that is, how the experiments are actually done. What people end up seeing is what biotech companies that want you to buy their $1000+ electrophoresis setups are saying.
I hope that in showing how we do DNA electrophoresis in our lab (at the ivoriest of all towers, harvard) I can show how experimental biology is often hacked together and DIY (which makes it really fun and sometimes frustrating) and has a lot more to offer than the final reports of what we make of the experimental results, and way more to offer than the bio-rad website. I'm not going to go through all of the DIYbio proposals on how to do gels, because the discussion boards listed above already go into good detail about what would work and what wouldn't and why. My goal is just to show that the way that we do it is already pretty cheap and is actually quite similar to some of the final proposals for the DIY gel box from Pearl Biotech.
So here's my method for making DNA gels, illustrated with some fancy cell phone camerawork. First, I weigh out some agarose, mix it with saline, and microwave it for two minutes. Agarose is relatively expensive at $420 for 500 grams, but you only need 1 gram per gel so it should last for a long time.
While the agarose is microwaving I put together my gel tray. We use a simple piece of plastic put together for us by the harvard machine shop wrapped with masking tape (really) around the bottom so that the melted agarose doesn't fall out. A small plastic comb makes the wells where I'll put the DNA.
Now my agarose is melted, and here comes the tricky part. We add ethidium bromide here, a super toxic, absolutely (maybe) will kill you DNA stain that allows us to see where the DNA is. This stuff is difficult to dispose of safely, and there are some other options for DNA stains that are more expensive, but probably better if you're going to be doing this someplace that doesn't have OSHA people all over it. 
Now I've poured my gel into my prepared gel tray and let it harden, it's time to load my DNA.

I put it into a plastic box, also made by the machine shop. This box is filled with the same saline solution I used to dissolve my agar, and has a wire running through it that can be connected to any power source. I mix the DNA with a solution of glycerol and a dye. The dye lets you see how far your sample has run through the gel, and the glycerol is heavier than water so it lets the DNA fall into the wells that the comb made in the gel.

After running a voltage through the gel for about 20 minutes the gel is ready to look at under UV light. This makes the ethidium bromide glow where it has bound to the DNA and you can take a picture of the gel.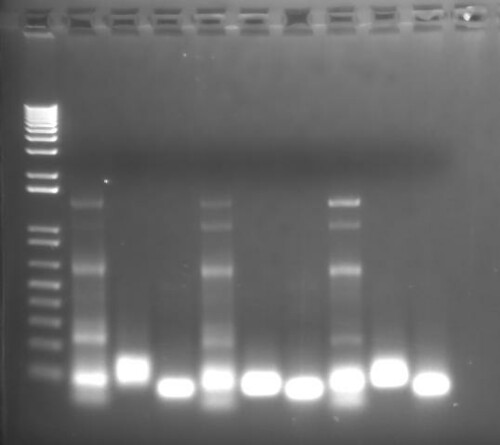
Tada! I hope that this shows that lab scientists and home scientists have a lot in common when we have similar goals, in this case separating out some DNA. I think if what people saw about DNA wasn't on CSI, Gattaca-like tests that gave you statistically suspect percentages for disease risk, or $500 canvases with DNA fingerprints that people wouldn't think scientists are so weird, and maybe would be even more interested in talking about biology at a bar. If you think this is a good idea, let us know. If you think it's boring, let us know; we can go back to talking about Star Trek and poop.
Sunday, April 19, 2009
Demystifying DNA electrophoresis
Posted by
Christina
at
6:22 PM
![]()
![]()
Subscribe to:
Post Comments (Atom)




No comments:
Post a Comment